Disclaimer & Copyright Notices; Optimized for the MS Internet Explorer
It is now clear that phosphorus is most often the limiting nutrient and that carbon or nitrogen is only briefly or rarely the limiting nutrient (Vollenweider, 1968; Likens, 1972; Schindler, 1974). While this emphasis on phosphorus has been very useful, it has often restricted the view of eutrophication and its control to simply that of external nutrient income and algal biomass. The problem is more complicated because most lakes and many reservoirs are small or shallow, with extensive littoral zones, macrophyte development, and high ratios of bottom sediment to lake volume. We now know that lakes and reservoirs contain interacting food webs and dynamic stores of nutrients in their bottom sediments that interact with the water column. They are not simply reaction vessels containing nutrients and algae. Managing and restoring lakes and reservoirs must include a recognition of the significance of littoral zones and macrophyte development, as well as the roles of biological interactions and feedback processes, which might combine to maintain high nutrient concentrations and plant biomass long after nutrient diversion.
Widely quoted restoration methodologies together with select case histories have already been summarized in our Synopsis #7 and Synopsis #8. The following have not been explained there.
Many lakes have extensive littoral areas, and the production of organic matter by these zones is very significant in the cycling of nutrients and in the nutrition of lake organisms. In particular, the large annual production of rooted plants and attached algae may be a major source of decomposing organic matter, and thus nutrients, to the open water and to the sediments of the hypolimnion. Not only are the sediments of the littoral zone the source of nutrients for these rooted plants, the plants may release nutrients to the water column via aerobic decomposition.
The pelagic zone, or open water area, is characterized by freely floating organisms-the zooplankton and phytoplankton-and by certain fish species. In some cases it appears that the nuisance algal blooms may be due as much to the absence of zooplankton grazing on them, following planktivorous fish feeding, as to the abundance of nutrients in the water.
The benthic community, the organisms living in and on the bottom sediments, may have a substantial community of insects, molluscs, and oligochaetes, although in eutrophic lakes these are reduced to a few low-oxygen-tolerant species. The dominant or- ganisms are microbes that convert dissolved and particulate organic matter into energy for themselves and release nutrients that can be reused by plants when transported to upper, lighted waters.
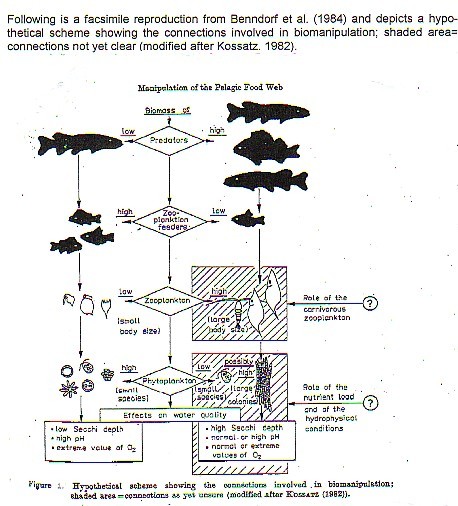
"Biomanipulation" includes lake improvement procedures that alter the food web to favor grazing on algae by zooplankton, or that eliminate fish species that recycle nutrients. Biomanipulation is new to the lake management community, particularly to those without training in limnology. There seems to be little question that the biomanipulation approach will become increasingly popular due its lower cost, and the absence of machinery and toxic chemicals (in some cases), and to its effectiveness. Biomanipulation involves eliminating certain fish species or restructuring the fish community to favor the dominance of piscivorous fish instead of planktivorous fish.
Food webs are controlled by resource limitation ("bottom-up") and by predation ("top-down"). Undoubtedly, solar energy and nutrient inputs and dynamics of an eco- system set its overall level of production, so to that extent the control may be envisaged as bottom-up, but within those limits, some of the "coarse-tuning" and much of the "fine- tuning" of structure and function in the system results from the complexity of top-down processes.
In the absence of fish predation, it is believed that large zooplankton predominate over small, due to their greater grazing efficiency and their ability to select particles over a wider range. Size-selective predation and size-efficiency hypothesis were apparently first formulated by Brooks and Dodson (1965) and are the basis of the fish planktivory- phytoplankton abundance portion of Shapiro's model. The biomanipulation model is also based upon several experiments demonstrating that benthivorous fish may be significant as internal sources of nutrients.
Case History #1 (Lynch and Shapiro 1981, 1982) conducted a series of experiments in Pleasant Pond, Minnesota. Twelve enclosures, 1.0 by 0.8 m, were suspended in deep water, ten with five different densities of planktivorous bluegill. All large-bodied zooplankton were eliminated from the fish enclosures. Algal volumes in fish enclosures were as much as 16 times those of fishless controls and were dominated by green algae. At the highest levels of fish predation a dense population of single filaments of Aphanizomenon flos-aquae developed, and Anabena circinalis bloomed. In fishless enclosures the phytoplankton biomass was small and dominated by flagellates and heavily armored species. Three levels of nitrogen and phosphorus enrichment were studied to test whether cycling of nutrients by fish could account for the algae bloom in fish enclosures. Algal volume remained higher in the enclosures that contained fish, supporting the conclusion that zooplankton removal and not nutrients was involved.
Case History #2 (Shapiro and Wright.1984); Round Lake, Minnesota (12.6 ha, max. depth= 10.5m, mean depth= 2.9m), a dimictic, eutrophic lake, was treated with rotenone to eliminate a fish community dominated by planktivorous bluegill and black crappie, and the benthivorous black bullhead, when the ratio had been about 165 planktivores to 1 of the piscivore. The lake was restocked with piscivorous largemouth bass and walleye in a ratio of 2.2 bluegills to 1 of the piscivorous fish. Channel catfish were added to prevent re-establishment of the black bullhead, an animal capable of producing a significant rate of internal nutrient loading. The mean Secchi disc transparency in the summer prior to rotenone application (1980) was 2.1 m. In summer 1981, following biomanipulation in September and restocking in October, 1980, the mean transparency was 4.8 m. In 1982 mean summer transparency was 4.7 m. Daphnia pulex, rare before treatment, became the dominant microcrustacean species, and it was found that herbivory by them can account for the improvement in transparency. A decline in total nitrogen and total phosphorus, which could not be accounted for by change in loading, occurred after biomanipulation. Wright and Shapiro hypothesize that the feeding of large Daphnia in the epilimnion at night, followed by descent to deep water during the day, produced a downward translocation of nutrients. If this is correct, then biomanipulation of this type may bring about lasting lake improvement.
Case History #3: (Wurtsbaugh and Li. 1993): In many lakes with planktivorous fish, however, large zooplankton continue to dominate. For this to occur, refuge from predation is necessary. In the case of eutrophic Clear Lake, California (170 km2 system, Zmax= 15m, mean SD= 1.2m), diel field studies indicated that the planktivores fed on zooplankton in the top 1-3m of the lake where light intensities were above 5x1012 photons/cm2.sec. Lab studies indicated maximum feeding rate near 7x1013 photons/cm2.sec (ca. 104 lux, or 10% of full sunlight), with the feeding rates near zero below light intensities of 1011 photons/cm2.sec (ca. 50 lux). The darker 5-15m strata of the lake allowed refuge for larger zooplankton, with many Daphnia greater than 1.5 mm being present. Such a refuge from predation may, in turn, reduce top-down control of food webs in productive lakes.
Case History #4: The use of rotenone can be difficult and expensive. Another option for restructuring a fish community is to introduce a piscivore. Benndorf et al. (1984) introduced perch and trout to a small, eutrophic quarry to control or eliminate planktivorous fish. Secchi disc transparency improved, even though phytoplankton biomass did not decline. This improvement was due to grazing, because the phytoplankton shifted to Chlamydomonas (with a high growth rate to compensate for grazing) and Oocystis (with a morphology that inhibits grazing).
Case History #5: Spencer and King (1984) have found ponds with fathead minnows and brook stickleback were dominated by intense algal blooms and had very low zooplankton biomass. A pond without fish and a pond with a dense population of large largemouth bass had very low phytoplankton biomass, but supported dense populations of submerged macrophytes due to the high water transparency.
Lynch and Shapiro (1981) concluded that phytoplankton in planktivore-free lakes will be less sensitive to enrichment if herbivores keep algal biomass below levels at which nutrients are critical. A lake's response to fish removal will depend upon the level of nutrient enrichment. In eutrophic, highly enriched lakes, in which phytoplankton are less sensitive or limited by nutrients, there will be higher sensitivity to changes in planktivory than to further nutrient enrichment. This suggests that lakes with large nutrient incomes, in which diversion is not possible or too expensive, would be good candidates for a fish removal or restructuring manipulation.
Case History #6 (Proulx et al. 1993): The effect of planktivorous fish on the phytoplankton community structure of an oligotrophic system (lac Croche at the Université de Montréal field station) was investi- gated with the help of large experimental enclosures under contrasting nutrient loading regimes. In both oligotrophic and eutrophic regimes total epilimnetic phytoplankton biomass increased in the presence of planktivory fish during summer stratification. Fish planktivory modified the algal size distribution by favoring the growth of small cells (2-10 µm). The taxonomic distribution was also influenced by the presence of fish. The fishless enriched enclosure were dominated by the green alga Schroederia, while the alga Scenedesmus dominated the fish fertilized enclosures. In the non-fertilized enclosure, the presence of fish favored hard scaled dinoflagellates.
Case History #7 (Lathrop et al. 1993): Means of Secchi disk readings were computed for spring turnover, early stratifica- tion, and stagnation periods (51-58 years) in eutrophic Lake Mendota, Wisconsin, USA. While decreased water clarity corresponded to increased nutrients only during summer stagnation, water clarity was significantly greater during years with high herbivory for all 3 seasonal periods, including the summer period when blue-green algal blooms occur.
If a lake is used primarily for recreational fishing, a choice may have to be made by lake users between algal blooms and high density of centrarchid fish on the one hand, and clear water on the other. The ideal solution may be to restructure the fishery for a balance of piscivorous and planktivorous fish, following a fish elimination.
Case Histories #8 (Benndorf & Miersch. 1991): A high reliability of biomanipulation (i.e. top-down control of eutrophication) could only be expected if the phosphorus loading a priori is below the threshold (oligotrophic and mesotrophic lakes), or if the phosphorus loading exceeding the threshold (eutrophic and hypertrophic lakes) will be reduced by other methods, or if the intensity of bottom-up mechanisms will be strongly controlled by light.
Comparison of top-down effects in 14 whole-lake biomanipulation studies (+ = effect observed; - = effect not observed; ? = no data):
Legend:
(a): Effect explained by external P-load reduction
(b): Shallow lakes with macrophytes
(c): High flushing rate of the water
Haugatjern:
L. Michigan:
Stockelidsvatten:
L. Trummen:
Tuesday L.:
Round Lake:
Lago di Annone:
Loch Loso:
Grafenhain:
Wirth Lake(b):
L. of Isles(b):
Broads Brundall:
Elbe backwaters:
Bautzen Reservoir:
There are four principal top-down effects which reduce in-lake phosphorus:
It must be emphasized that the P-increase effects are independent on the external and internal P-loading. But several of the P-decrease effects are strongly inhibited by high external and/or internal P-loadings. Thus, the overall effect of decreasing in-lake phosphorus as a consequence of biomanipulation was observed only in lakes having P-loadings below the threshold of about 0.6g total P/m2.yr. If the critical threshold of P-loading is exceeded, then high P-loading coupled with the suppression of the P-reduction mechanisms and with the relatively high intensity of P-increase mecha- nisms causes high (unchanged or increased) in-lake phosphorus and hence no reduction in phytoplankton biomass.
The validity of the proposed hypothesis of a "biomanipulation-efficiency threshold of the P-loading" must be checked by further independent whole-lake studies and enclo- sure experiments with various P-loadings as well as by model investigations.
Case History #9: Obviously, control or elimination of planktivorous fish in drainage lakes may be difficult or impossible, and may even be undesirable where the lake is used for fishing. Timms and Moss (1984) suggest an alternative to fish elimination in shallow lakes. They provide evidence that clear water in the nutrient-enriched Hudsons Bay was due to grazing by large-bodied zooplankton, which had escaped fish predation by taking refuge during the day in water lily beds. They suggest that it might be possible to mimic this refuge in macrophyte-free lakes by adding some type of artificial "structure" that could be used as a littoral refuge by zooplankton. This idea is similar to the use of aeration to open the dark hypolimnium as a refuge from fish visual predation.
In the enclosures without fish, as the high grazing by abundant mesozooplankton reduced nanoplankton biomass, large phytoplankton which usually cannot compete with pico- and nanoplankton, took advantage of available nutrients and increased in biomass. In these enclosures, the major fraction of PP was larger than 20 um, the fraction of total phosphorus (TP) sedimenting was higher and seasonal TP was lower. Nutrient, added or regenerated, was removed quickly via sedimentation.
In the enclosures with fish, abundant microzooplankton was associated with increased PP in pico- and nanoplankton, and plankton community had severe phosphate limitation. Nutrient, added or regenerated, was rapidly assimilated by pico- and nano- plankton. The major fraction of PP was in particles smaller than 20 µm, the fraction of TP sedimenting was small and seasonal TP was higher.
Flux of phosphorus from pico- and nanoplankton to mesozooplankton and total zooplankton communities via grazing was greater in the enclosures without fish. Grazer- mediated changes in size-distribution of plankton and in the competition for available phosphate between small and large phytoplankton, regulate the total biomass of plankton by influencing sedimentation.
Shapiro (1979) points out that agricultural runoff is often contaminated with significant amount of pesticides, which are extremely toxic to zooplankton, even in trace amounts. He lists the 48-hour LC50 to zooplankton is less than 1.0 uµg/l and notes several cases in which elimination of zooplankton by pesticides was associated with algal blooms.
Planktonic microcrustacea are not the only group of organisms that may alter algal density. Brabrand et al. (1983) suggest that Oscillatoria blooms could be controlled, at least in small lakes, by inoculation of the ciliated protozoan Nassula, particularly when copepod density is low.
We salute the Chebucto Community Net (CCN) of Halifax, Nova Scotia, Canada for hosting our web site, and we applaud its volunteers for their devotion in making `CCN' the best community net in the world